Science Highlight
OVOCs in the Remote Marine Atmosphere
The world’s oceans represent the largest surface organic carbon reservoir on the Earth and the dissolved organic matter within the sea-surface microlayer is known to produce a variety of low-molecular-weight VOCs and OVOCs. The photolysis of OVOCs directly impacts atmospheric oxidation by serving as additional sources of HOx radicals, as does the participation of highly chemically reactive aldehydes in remote atmosphere chemical cycles. Analyses of marine OVOC distributions from ATom TOGA measurements have to date focused on acetaldehyde (CH3CHO) (Wang et al., 2019), acetone (Wang et al., 2020), methanol (Bates et al., 2021), and methyl ethyl ketone (MEK) (Brewer et al., 2020). The tropospheric VOC profiles of all of these species exhibit strong vertical gradients. Acetone and methanol are two of the most abundant oxygenated volatile organic compounds (VOCs) in the atmosphere. Our measurements and analysis show that acetone may contribute up to 30–40% of HOx production near the tropopause, especially in the tropical regions, where troposphere‐stratosphere exchange is active (e.g., strong convective transport or Asian Monsoon). Background methanol concentrations (200-400 ppt) were found to be mostly controlled by secondary chemical production from the CH3O2 + OH and CH3O2 + CH3O2 reactions (global sources of 33 and 24 Tg a-1, respectively) providing for ≈ 30% of the global source of methanol. The CH3O2 + OH reaction is generally not included in global models of atmospheric chemistry, but the ATom results show that it produces methanol with 13% yield and, more broadly, impacts hydrogen oxide (HOx) radical budgets; thus it should be included in the models.
Because of its high reactivity and short lifetime, the ATom observations of acetaldehyde are particularly surprising and also consequential. This species is a significant contributor to the OH reactivity and may be a critical component of halogen cycles in the marine boundary layer (MBL) and free troposphere (Figure 1a). Concurrent observations of peroxyacetic acid (PAA), a product of CH3CHO oxidation, demonstrate a similar MBL enhancement. Using an observationally constrained box model, Wang et al. (2019) showed that the observed CH3CHO can explain the observed PAA, providing support for the veracity of both measurements (Figure 1b,c). Standard 3D model simulations, e.g., the Community Atmosphere Model with Chemistry (CAM-chem, Wang et al., 2019) and GEOS-Chem (Travis et al., 2020) severely underpredict CH3CHO in the remote global troposphere. The addition of both an oceanic flux parameterization for CH3CHO and a potential source of CH3CHO from organic aerosols considerably improved the agreement between the model results (Wang et al., 2019, Travis et al., 2020) and observations but there is still an unaccounted CH3CHO source in the remote troposphere (measurements still lower than models even with the proposed sources, Fig 1b).
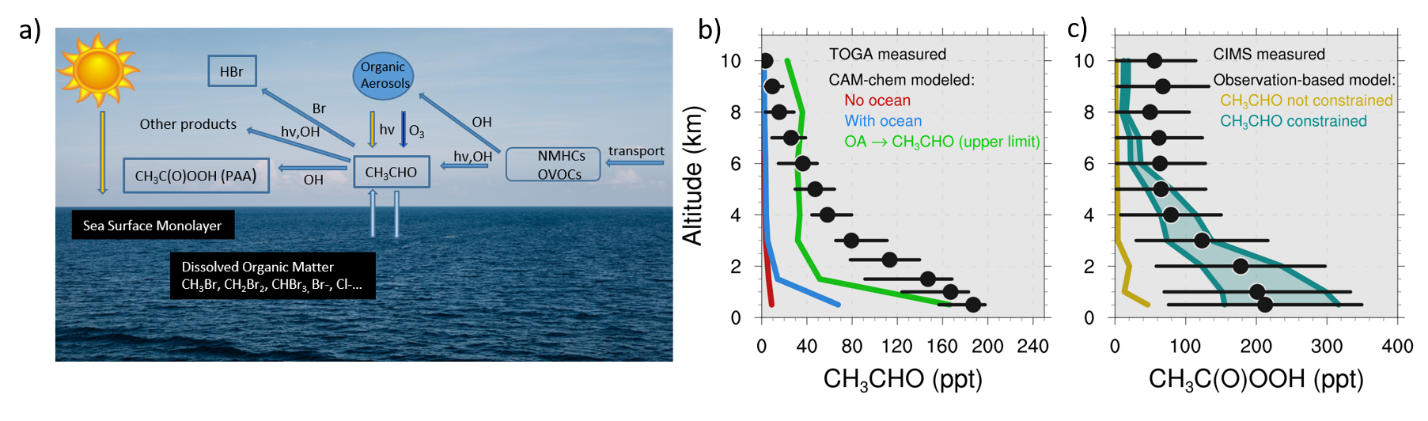
Figure 1. Processes involving acetaldehyde in the MBL (a). Altitude profiles of (b) CH3CHO and (c) CH3C(O)OOH (PAA) over the remote Pacific during ATom-1. Altitude-binned observations are shown in black with 25th and 75th percentiles shown as black bars.