Chamber experiments to study gas phase chemical mechanisms and to evaluate instrumental capabilities to measure volatile organic compounds (VOCs) in both low and high NOx regimes
Experiments conducted in atmospheric simulation chambers have played a large role in elucidating chemical processes that occur in the atmosphere by simplifying the matrix yet retaining the important reactants and co-factors that influence the outcomes of photochemical reactions in the real world. ACOM has a dedicated 10-m3 environmental chamber which is used both for this purpose and to test and compare instruments side-by-side to evaluate their analytical capabilities and limitations. The majority of atmospheric chemistry experiments conducted using chambers have been done at relatively high values of atmospheric nitrogen oxide(s) (NOx) concentrations. There is a great deal of current interest in lower NOx regimes where NOx < 100 ppt because emissions technologies are driving ambient NOx levels downward at a high rate. Atmospheric chemical reactions can take different pathways depending on the amount of NOx available. We recently investigated the oxidation pathways of n-butane (nC4H10) and 1-butene (1-C4H8), as well as isoprene (C5H8) in high -and low-NOx environments using the NCAR Trace Organic Gas Analyzer (TOGA) and a Proton Transfer Reaction Mass Spectrometer PTR-MS. Our data suggest that C4 alkene and alkane oxidation through the HO2 pathway is analogous to isoprene, and that C4 hydroperoxide oxidation products are also susceptible to decomposition within commercial instruments. A conclusion from this study is that any instrument deployed in low NOx environments should be tested and evaluated for hydroperoxide artifact interference with OVOC measurements prior to deployment. We show that, in contrast to PTR-MS instruments, TOGA has minimal instrument bias with respect to OVOCs in low-NOx environments due to the design of its introduction system and choice of wetted material. This feature of the TOGA instrument is allowing us to study the distributions of oxidation products in the low-NOx environments encountered during the NASA Atmospheric Tomography (ATom) mission and other field campaigns involving low-NOx regimes. Figure 1 shows results from the isoprene portion of the experiment that demonstrate these effects.
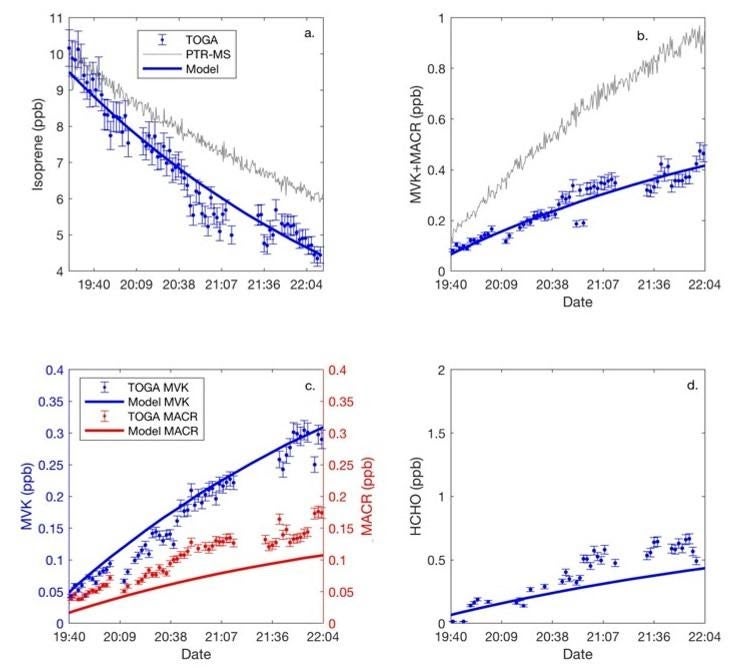
Figure 1. Depiction of VOC reactant isoprene's photochemical loss throughout a chamber experiment under low-NOx conditions, and concurrent accumulation of OVOC products methacrolein (MACR), MVK, and formaldehyde (HCHO). The large excursion of the PTR-MS data from the box-modeled and TOGA MVK+MACR data in (b) indicate that the PTR-MS has a large artifact when measuring isoprene oxidation products in low-NOx environments whereas TOGA does not. Missing data in the TOGA time series are from times that TOGA sampled non-chamber air.