Reductions in Anthropogenic Carbon Monoxide Emissions Lead to Shorter Methane Lifetimes in the Atmosphere
About half of atmospheric carbon monoxide (CO) is from direct (CO) emissions that are due to incomplete combustion and are related to both natural (e.g. wildfires) and anthropogenic activities. The remainder of CO in the atmosphere is produced from the chemical oxidation of hydrocarbons, mainly from biogenic sources and methane (CH4). Since most of the hydrocarbons, CO, and CH4 in the atmosphere are oxidized by the hydroxyl radical (OH), the associated chemical lifetimes of these species are strongly coupled with OH. For example, a reduction in CO emissions leads to more OH and therefore a shorter CH4 lifetime, assuming CH4 emissions are the same. This means that long-lived greenhouse gases such as CH4 are linked with short-lived pollutants related to air quality. We use the NCAR coupled chemistry-climate model of the Community Earth System Model (or CESM http://www.cesm.ucar.edu/) Community Atmospheric Model with Chemistry (or CAM-Chem) to investigate the coupled nature of our chemical system through a decadal chemical reanalysis experiments. We assimilate weather observations and satellite-based measurements of CO from The Measurement of the Pollution in the Troposphere (MOPITT) across the recent decade (2002-2013) using the Data Assimilation Research Testbed (DART, http://www.image.ucar.edu/DAReS/DART/).
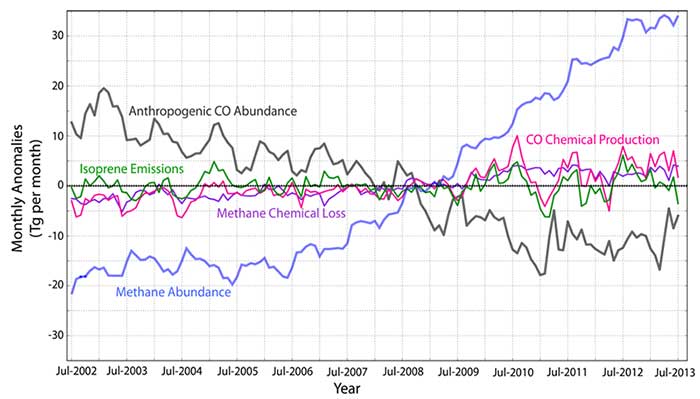
Based on these experiments, we confirm previous reports of decreasing trends in global CO burden that is mainly attributed to the reduction of CO emissions from anthropogenic sources. However, the impact of this reduction in CO on CH4 chemical loss is not clear at this juncture. The rate of CH4 loss is dependent upon CH4 abundance and OH concentrations. It is also evident from atmospheric CH4 observations that the increase in CH4 sources leads to a positive growth rate of CH4 abundance over the recent decade and therefore leading to a positive trend in the absolute magnitude of CH4 chemical loss. As a consequence, this increase in CH4 loss leads to a positive trend in the chemical production of CO (secondary CO). From our reanalysis, we find that the other sources of secondary CO from biogenic sources (represented here as isoprene which is most important hydrocarbon emitted from vegetation), exhibits no significant trend. Since the chemical lifetime of CH4 is inversely proportional to OH (where its main sink is CO), we infer from our analysis that during this period of long-term decline of CO abundance (mainly from changes in anthropogenic CO emissions), it is likely that we observed a reduced rate of increase in CH4 abundance and loss than in a scenario where CO emissions are the same. These results show the potential cobenefits of reducing both CO and CH4 emissions to mitigate climate and air pollution impacts.
Reference: Gaubert, B., Arellano, A. F., Barré, J., Worden, H. M., Emmons, L. K., Tilmes, S., Buchholz, R. R., Vitt, F., Raeder, K., Collins, N., Anderson, J. L., Wiedinmyer, C., Martinez Alonso, S., Edwards, D. P., Andreae, M. O., Hannigan, J. W., Petri, C., Strong, K., and Jones, N.: Toward a chemical reanalysis in a coupled chemistry-climate model: An evaluation of MOPITT CO assimilation and its impact on tropospheric composition, J. Geophys. Res.-Atmos., doi:10.1002/2016JD024863, http://dx.doi.org/10.1002/2016JD024863, 2016JD024863, 2016.