A 1D-model study of the role of snow in controlling halogen chemistry in the springtime Arctic boundary layer (OASIS).
In recently submitted work (Ahmed et al., 2022) led by colleagues at Université de Grenoble Alpes (Shaddy Ahmed, in the group headed by Jennie Thomas), and involving numerous ACOM co-authors, we report on the application of a 1-D atmospheric model to the study of halogen chemistry and surface ozone depletion in the springtime Arctic. More specifically, the project employed data from the OASIS (Ocean-Atmospheric-Sea Ice-Snowpack) 2009 ground-based field campaign held at Utqiagvik Alaska aimed at elucidating the physico-chemical processes involved in springtime boundary layer ozone depletion events (ODEs) that occur sporadically in the Arctic. OASIS measurements were made by researchers from ACOM and numerous Universities, and included ozone, particulates, inorganic halogens, NOx and HOx species, actinic flux, and VOCs.
Partial and complete ODEs, accompanied by the presence of inorganic bromine, were prominent during the campaign (March-April, 2009), as had been observed on previous occasions. What was not expected was the presence of high levels (up to hundreds of pptv) of Cl2 observed by the Georgia Tech group (Liao et al., 2014). This surprising observation triggered questions about the source of the Cl2, and the vertical extent of its impacts on boundary layer chemistry. In the Ahmed et al. study, the PACT-1D (Platform for Atmospheric Chemistry and Vertical Transport in 1-dimension) model, co-developed at UCLA and Grenoble (Tuite et al., 2021), was extended to include direct emission of Cl2 and Br2 from the surface, as well as a ‘recycling’ source of these gases, and was refined to match OASIS observations. Some key findings are highlighted below:
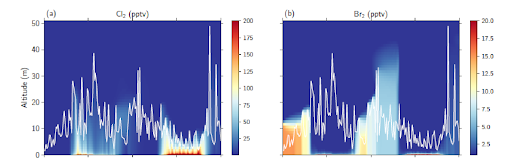
- Observations of Cl2 near the surface from OASIS-2009 could be reproduced by the model, with primary emissions dominating. The vertical extent of the Cl2 was limited to only the first 15 m above the surface on the two model days investigated; thus, while impacting strongly the oxidative chemistry near the surface, these impacts decrease dramatically at higher altitudes. See accompanying figure.
- Modeled levels of inorganic bromine were also well represented by the model, with both primary and recycling sources of Br2 of importance.
- Recycling of inorganic halogens on aerosols was found to be at best a minor contributor to the overall chemistry.
References:
Ahmed, S., et al., (2022) The role of snow in controlling halogen chemistry and boundary layer oxidation during Arctic spring: A 1D modelling case study, J. Geophys. Res., under review. (preprint available at: https://www.essoar.org/doi/abs/10.1002/essoar.10508675.1)
Liao, J., et al., (2014). High levels of molecular chlorine in the Arctic atmosphere. Nature Geoscience, 7 (, 91–94. doi: 10.1038/ngeo2046
Tuite, K., et al., (2021). Quantifying nitrous acid formation mechanisms using measured vertical profiles during the CalNex 2010 campaign and 1D column modeling. J. Geophys. Res. Atmos., 126, e2021JD034689. doi: 10.1029/2021JD034689