MOZART Chemical Mechanism Development
The family of MOZART (Model of Ozone and Related chemical Tracers) chemical mechanisms are used in the Community Earth System Model (CESM), including CAM-chem and WACCM, as well as in WRF-Chem. The chemical mechanisms released in CESM2 are the TS1 (troposphere and stratosphere) mechanism for CAM-chem, and the TSMLT1 (troposphere, stratosphere, mesosphere and lower thermosphere) mechanism used in WACCM [Emmons et al., 2020; Gettelman et al., 2019]. A secondary organic aerosol (SOA) scheme using a volatility basis set (VBS) parameterization, is coupled with the standard Modal Aerosol Model and used in both CAM-chem and WACCM [Tilmes et al., 2019]. The CESM2.2 release includes the MOZART-TS2 mechanism, which has an expanded representation of isoprene and terpene oxidation [Schwantes et al., 2020]. Isoprene and terpenes are biogenic hydrocarbons that are emitted from vegetation. During the summer in the southeastern U.S. where there are high biogenic emissions, many regional and global models are biased high for surface ozone compared to observations, and we found that the MOZART-TS2 chemical mechanism greatly reduces that bias [Schwantes et al., 2020]. The MOZART-TS2 mechanism is of similar complexity to other recently updated mechanisms for isoprene, but is significantly more complex than the terpene oxidation mechanisms used in many regional and global models. Further expansion of the tropospheric mechanism is in progress, in particular for more explicit representation of larger alkanes and their oxidation products (MOZART-TS3). We are also developing a simpler mechanism, similar to MOZART-2, which will be suitable for climate simulations.
The MOZART-TS3 mechanism provides a better representation of larger alkanes and their products in the remote atmosphere in comparison to measurements made with the TOGA instrument during the ATom aircraft campaign. For example, the median vertical profile of methyl ethyl ketone (MEK) during ATom-1 is shown in Figure 1 with observations from TOGA shown in black. MEK is overestimated in CESM/CAM-chem using the MOZART-TS1 chemical mechanism (red), which groups all C4 and greater ketones as MEK. With the addition of more complex isoprene and terpene chemistry (gold) and especially alkane chemistry (purple), simulated MEK is much closer to the observations. The global impacts of using chemical mechanisms of varying complexity (e.g., simulated HOx in the marine boundary layer and upper troposphere) are also being explored in this work. A manuscript for this analysis is in preparation [Schwantes et al., in prep.] and this work has been presented at two conferences [Schwantes et al., ATom STM, 2019; Schwantes et al., AGU, 2019].
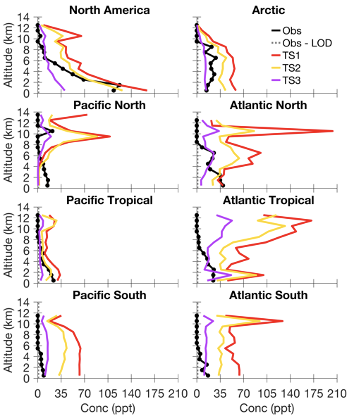
Figure 1. Methyl ethyl ketone (MEK) median vertical profiles separated by region for ATom-1 TOGA observations (black) and CESM/CAM-chem sampled over the ATom flight tracks for different chemical mechanisms of varying complexity: default chemical mechanism in CESM/CAM-chem (red), updated isoprene and terpene chemistry (gold), and updated isoprene, terpene, and alkane chemistry (purple).
References
Emmons, L.K., Schwantes, R. H., Orlando, J. J., Tyndall, G., Kinnison, D., Lamarque, J. -F., Marsh, D., Mills, M., Tilmes, S., Bardeen, C., Buchholz, R. R., Conley, A., Gettelman, A., Garcia, R., Simpson, I., Blake, D. R., Meinardi, S., Pétron, G. (2020), The Chemistry Mechanism in the Community Earth System Model version 2 (CESM2), J. Advances in Modeling Earth Systems, 12. https://doi.org/10.1029/2019MS001882
Gettelman, A., Mills, M. J., Kinnison, D. E., Garcia, R. R., Smith, A. K., Marsh, D. R., et al. (2019). The whole atmosphere community climate model version 6 (WACCM6). Journal of Geophysical Research: Atmospheres, 124. https://doi.org/10.1029/ 2019JD030943
Tilmes, S., Hodzic, A., Emmons, L. K., Mills, M. J., Gettelman, A., Kinnison, D. E., et al. (2019). Climate forcing and trends of organic aerosols in the Community Earth System Model (CESM2). Journal of Advances in Modeling Earth Systems, 11. https:// doi.org/10.1029/2019MS001827
Schwantes, R. H., Emmons, L. K., Orlando, J. J., Barth, M. C., Tyndall, G. S., Hall, S. R., Ullmann, K., St. Clair, J. M., Blake, D. R., Wisthaler, A., and Bui, T. P. V.: Comprehensive isoprene and terpene gas-phase chemistry improves simulated surface ozone in the southeastern US, Atmos. Chem. Phys., 20, 3739–3776. https://doi.org/10.5194/acp-20-3739-2020, 2020
Schwantes, R. et al., More Explicit Alkane Chemistry Improves the Representation of Several Volatile Organic Compounds Including Methyl Ethyl Ketone in the Remote Atmosphere, Poster Presentation, ATom Science Team Meeting, Boulder, Colorado, November 2019.
Schwantes, R. et al., More Explicit Alkane Chemistry Improves the Representation of Several Volatile Organic Compounds Including Methyl Ethyl Ketone in the Remote Atmosphere, Oral Presentation, American Geophysical Union, Fall Meeting, San Francisco, CA, December 2019.
Schwantes, R. H., Orlando, J., Hornbrook, R. S., Apel, E. C., Hills, A., Wang, S., Emmons, L. K., et al., More Explicit Alkane Chemistry Improves the Representation of Propanal, Acetone, Butanal, Methyl Ethyl Ketone, and HOx in the Remote Atmosphere, in prep., 2020.