Gas-Phase Dry Deposition as a Major Removal Mechanism for Secondary Organic Aerosols (SOA)
Removal of secondary organic aerosols (SOA) from the atmosphere has been studied far less than its equal, production. In current regional and global chemistry models rainout is the dominant loss of SOA. Here we show the importance of a less direct pathway, in which large scale evaporation of SOA particles occurs as a re-adjustment to gas-particle partitioning when semi-volatile organic gases are lost by dry deposition to the Earth’s surface.
The formation of SOA particles is coming to be fairly well understood – at least semi-quantitatively – as resulting from the condensation of low-volatility partly oxidized organic compounds that are intermediates in the atmospheric oxidation of many hydrocarbons, both biogenic and anthropogenic. This complex chemistry is being mapped with the Generator of Explicit Chemistry and Kinetics of Organics in the Atmosphere (GECKO-A) model built collaboratively at NCAR and the University of Paris. The GECKO-A model uses chemical reaction pathways and their kinetics from compilations of laboratory measurements where possible, and extends these with estimations based on structure-activity relations for molecules where no measurements exist. Typical chemical mechanisms spawned by GECKO-A contain several million reactions among many hundreds of thousands of species. The explicit chemical nature of the molecules is retained, and can be used to estimate additional properties including chemical reactivity, optical properties, and thermodynamic properties such as boiling points, vapor pressures (C*), and Henry’s law solubility coefficients (H). Correlations between C* and H are shown in Figure 1, each panel representing the molecular daughters of different precursor hydrocarbons.

Figure 1.
For condensable species, the concentrations in the gas and particle phase, g and p, are related by
g/p =C*/OM Eq.1
where OM is the total particle-phase organic mass and C* is the saturation vapor pressure (in mass units). From Figure 1, it is seen that for typical conditions (M ~ 0.1 – 10 mg m-3) species with C* in this range (the gray region of the panel) will have substantial mass in both gas and particle phases.
The partitioning Equation (1) has implication also for the removal of SOA. Dry deposition is usually considered to be much slower for particles than for gases (because diffusion through the surface layer scales with the reciprocal square root of mass). But Eq. 1 suggests that dry deposition of gases, which can be fast in many circumstances, will cause evaporative adjustment of the particle mass. Thus, careful accounting of gaseous dry deposition is essential to predict the evolution of particle mass as well.
A key parameter for the estimation of gaseous dry deposition is the Henry’s law solubility coefficient. “Dry” deposition actually refers to the deposition of a (dry) gas onto a surface which is often wet (e.g. surface waters, stomata and moist vegetation), and so the Henry’s law solubility often determines the fate of the molecules striking the surface. Using GECKO-A, we were able to calculate Henry’s law solubility coefficients for the myriad of semi-volatile organic products, as shown in Figure 1, and showed that they correlate well with C*. These correlations were implemented in a regional chemistry model, WRF-Chem, to examine the influence of SOA dry deposition. The results are shown in Figure 2, separately for anthropogenic and biogenic hydrocarbon precursors. For the case studies, dry deposition is the largest loss pathway for SOA, and shows that a large fraction of the SOA is removed well before encounter with cloud and eventual rainout. Averaged over the contiguous U.S., dry deposition accounts for about a factor of two reduction in surface SOA particle concentrations, while wet deposition is far less important than previously assumed. A paper describing the work is in press.
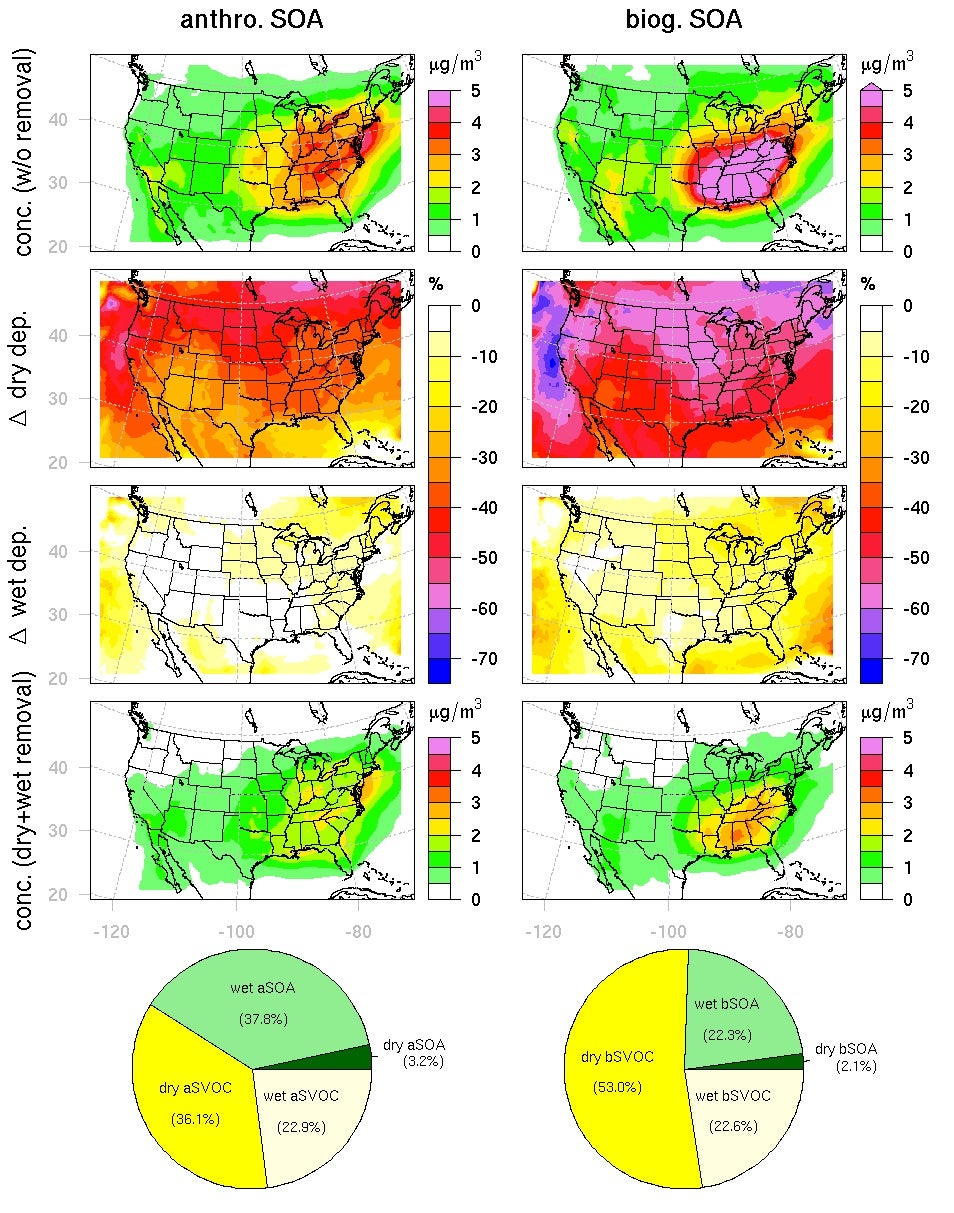
Figure 2.
References:
Hodzic, A., B. Aumont, C. Knote, J. Lee-Taylor, S. Madronich, and G. Tyndall (2014), Volatility dependence of Henry’s law constants of condensable organics: Application to estimate depositional loss of secondary organic aerosols, Geophys. Res. Lett., 41, doi:10.1002/2014GL060649.
