Exploring Methane Oxidation Through Hydrogen Isotopes
In collaboration with researchers from the State University of New York’s College of Environmental Science and Forestry in Syracuse, ACD scientists have measured the fractionation of deuterated methoxy radicals, CH2DO, which are formed in the atmospheric oxidation of methane. Methane, CH4, is the most-abundant hydrocarbon in the atmosphere, as a result of its large source strength and low rate of reaction with OH radicals. Its oxidation provides a global background sink for OH and a source of CO. The methane lifetime is sufficiently long, ~10 years, that a fraction of it is transported into the stratosphere, where its oxidation leads to the production of water vapor. This in-situ production of water adds to the small amounts of water that are transported up through the tropopause. Thus, understanding the global cycle of methane oxidation is important both for a quantitative discussion of oxidizing capacity and climate. The oxidation of methane leads, in part, to the production of hydrogen gas, H2, via the photolysis of formaldehyde, HCHO (see Figure 1 below). Our current understanding of the budget of H2 in the atmosphere is not complete, and with the possibility of increased future use of hydrogen as an alternative fuel source, understanding the H2 budget takes on added importance.
Measurements of the isotopic composition of hydrogen in the atmosphere have shown an enrichment in the heavy isotope (deuterium) which has been suggested to come from the oxidation of deuterated methane (CH3D).1 As seen in the reaction scheme below, enrichment in the heavy isotope can occur during the initial reaction with OH radicals, or from the production of formaldehyde from the methoxy radical CH2DO.
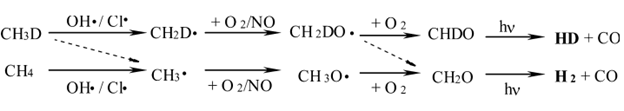
The laboratory experiments were conducted by ESF graduate student Hongyi Hu, who is working for her Ph.D. with Professor Theodore Dibble, in the laboratory of Geoff Tyndall and John Orlando in ACD. The experiments involved the photolysis of singly deuterated methyl nitrite, to produce the radicals, which then reacted with O2 to form either HCHO or HCDO. The loss of starting material and formation of the products were both monitored by Fourier Transform Infrared Spectrometry.
CH2DONO + hv → CH2DO + NO
CH2DO + O2 → HO2 + HCDO (rate k1a)
CH2DO + O2→ DO2 + HCHO (rate k1b)
Measurement of the ratio of HCDO/HCHO then gives a direct measure of the ratio of the rate constants, k1a/k1b. The technique had previously been used to measure the ratio at room temperature in a study by workers at Ford Motor Company and the University of Copenhagen.2 Ms. Hu characterized the ratio over a much wider temperature range during 2 visits to NCAR. In addition to synthesizing the CH2DONO, running the experiments, and analyzing the data, she also carried out kinetic modeling of the system to ensure that side reactions were suppressed or accounted for. The results are summarized below.
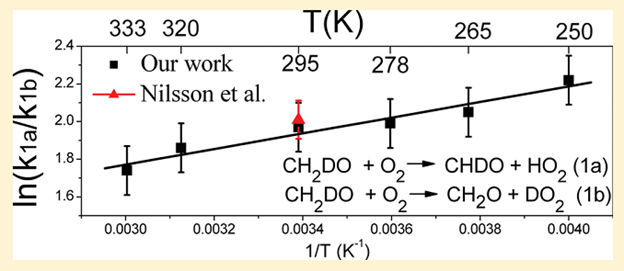
The results indicate that the isotope fractionation is much larger at low temperature than at room temperature, which will assist the modeling of the HD/H2 ratio in the stratosphere. The work is fully described in a recently published paper in the Journal of Physical Chemistry A.3 Now back at ESF, Hongyi is working on a theoretical treatment of the reaction rate, in order to:
a) better understand the sources of the kinetic isotope effect
b) reliably extend the experiments to relevant temperatures lower than achievable in the laboratory
c) validate a quantum chemical method to be used to study the reactions of other alkoxy radicals with O2.
The validated quantum chemical method would be extremely helpful for understanding the degradation pathways of alkoxy radicals formed in the atmospheric oxidation of many volatile organic compounds (VOCs), such as oxygenated VOCs and isoprene.
References
1. Rahn, T., J. M. Eiler, K. A. Boering, P. O. Wennberg, M. C. McCarthy, S. Tyler, S. Schauffler, S. Donnelly, and E. Atlas, 2003: Extreme deuterium enrichment in stratospheric hydrogen and the global atmospheric budget of H2, Nature, 424, 918-921.
2. Nilsson, E. J. K., M. S. Johnson, F. Taketani, Y. Matsumi, M. D. Hurley, and T. J. Wallington, 2007: Atmospheric deuterium fractionation: HCHO and HCDO yields in the CH2DO + O2 reaction, Atmos. Chem. Phys. 7, 5873-5881.
3. Hu, H., T. S. Dibble, G. S. Tyndall, and J. J. Orlando, 2012: Temperature-dependent branching ratios of deuterated methoxy radicals (CH2DO) reacting with O2, J. Phys. Chem. A., 116, 6295-6302.
