Understanding the Role of Cloud Chemistry by Using WRF-Chem Model Trajectories to a Mountain Measurement Site
Since the amendments to the Clean Air Act in the 1990s and 2000s were enacted, decreases in emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) have led to significant decreases in the acid deposition across the United States. At Whiteface Mountain in upstate New York, the acidity of clouds and the major ions contributing to the acidity, such as sulfate, nitrate, and ammonium, have been monitored for decades. The measurements at Whiteface Mountain have documented the increase of cloud water acidity from very acidic conditions to conditions now that represent clean, background air. With sulfate concentrations decreasing, the contribution of organic acids to the acidity of cloud water has become more important.
In an effort to understand how organic acids are formed in both the gas phase and cloud water, ACOM in collaboration with the University of Albany have developed a modeling system analysis framework with the Weather Research and Forecasting model coupled with chemistry (WRF-Chem). The system first runs WRF-Chem, launching trajectories that may reach Whiteface Mountain. The starting locations of the trajectories are either determined from past weather simulations or are set to a prescribed location. Here, we share results of trajectories launched from Pinnacle State Park in southern New York (shown in panel a) of the figure), where air quality measurements are also taken. The WRF-Chem trajectories gather WRF-Chem results of the meteorological state, chemical and aerosol composition, and other information such as photolysis rate constants. This information is then employed in the gas-phase chemistry box model BOXMOX to allow more detailed analysis of the chemistry along the air parcel trajectory.
An important organic acid is formic acid (HCOOH) which is formed from biogenic and anthropogenic alkenes in the gas phase and from formaldehyde and other organic acids in the aqueous phase. Shown in panel a) of the figure are the HCOOH mixing ratios predicted by BOXMOX along 40 trajectories launched at either 100, 200, or 500 meters above ground for over a 6.5-hour period from Pinnacle State Park. Many of the BOXMOX calculations show increases of HCOOH along the trajectories, especially the ones that veer south-eastward from upstate New York. In panel b), the mean HCOOH mixing ratios show strong increases during the first 12 hours of BOXMOX calculations. This is in contrast to the WRF-Chem predictions of HCOOH that show substantial decreases in HCOOH during the first 20 hours of the trajectory. Note that BOXMOX is calculating only gas-phase chemistry while WRF-Chem is calculating gas, aerosol, and cloud chemistry and includes wet and dry deposition. Thus, the differences between WRF-Chem and BOXMOX are due to encounters with precipitating cloud that removes HCOOH. Nevertheless, it is instructive to examine the BOXMOX gas-phase production of HCOOH along the trajectories, which shows the importance of ethene (C2H4), isoprene, and methacrolein (MACR) during the first 12 hours, and acetylene (C2H2) for a few hours on the second day (panel d). Acetylene emissions map for the trajectories is shown in panel c).
During the next year we expect to implement cloud chemistry in BOXMOX as well as use the much more detailed gas and aqueous-phase chemistry parcel model CAPRAM developed at TROPOS in Leipzig, Germany. We also hope this innovative analysis technique can be applied to other atmospheric chemistry measurement sites.
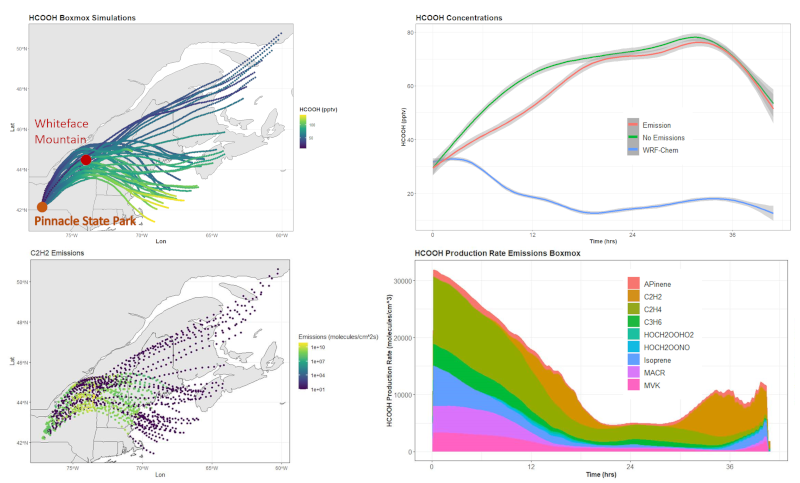
Figure 1. Results from BOXMOX chemistry box model. Panel a) shows trajectories, generated by WRF-Chem, with BOXMOX predictions of formic acid (HCOOH). Panel b) shows mean HCOOH (pptv) predicted by BOXMOX of 40 trajectories (shading shows range of results) with emissions (red line) and without emissions (green line) included. Also shown is the mean HCOOH predicted by WRF-Chem of the 40 trajectories. Panel c) shows trajectories with the emissions of acetylene (C2H2) emissions (molecules cm-2 s-1) that were used for the BOXMOX calculations with emissions. Panel d) displays the major oxidation reactions forming HCOOH in the BOXMOX calculations. The colors are labeled according to the volatile organic compound being oxidized. Click for larger image.